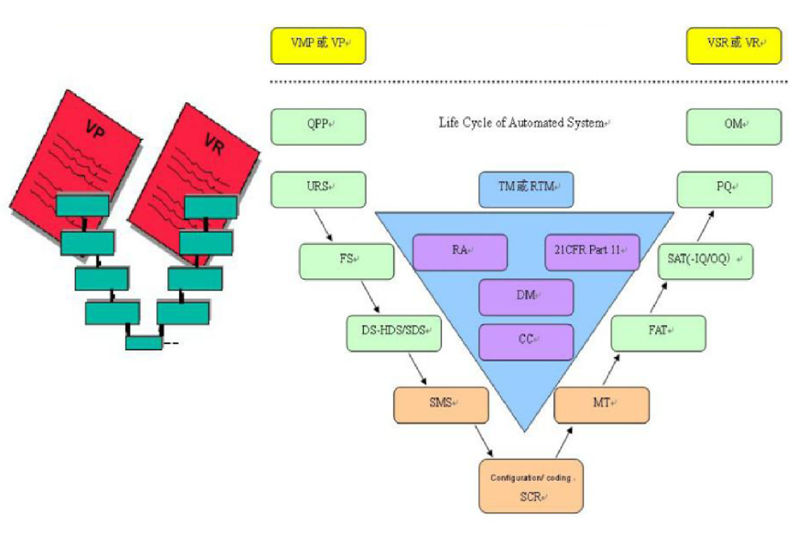
V-Module valaidation period and document framework
When Shanghai Ritai supplies high quality equipment, we also provide valaidation support for clients, including DQ, IQ, OQ, and necessary PQ. The valaidation support of Ritai acts as per V Module. For high quality valaidation requirement project, we can provide more complete and perfect valaidation service. We provide all documents of project based on our QMS:
●Quality and project planRegulatory requirements for pharmaceutical process systems during verification and testing. Our full set of verification systems and documents are based on FDA, EU GMP, and Chinese cGMP requirements. They mainly include and not only include:
●Validation Plan